Introduction
The Gunungkidul waters serve as a habitat for various commercial fish species and have contributed significantly to the economic growth of the local community, particularly for the 2,276 local fishers. The monthly local income for each fisher was about 150 to 300 USD, and most fishers earned only from fishing (Tirtadanu et al., 2024a). One of the commercial fish species in Gunungkidul waters is the giant catfish (Netuma thalassina), with production reaching 127 tons in 2023, with an estimated economic value of 121,275 USD (MMAF, 2024).
The giant catfish fishery has grown intensively as lobster production in Gunungkidul waters declines due to over-exploitation (Tirtadanu et al., 2022). The transition from lobster traps to other fishing gears, such as bottom longline and surface gillnet, is linked to the decline of the lobster stocks. Lobster production fell from 63 tons in 2019 to 20 tons in 2023. In contrast, marine catfish production has increased from 13 tonnes in 2019 to 420 tonnes in 2023 (MMAF, 2024). Despite the higher price of lobster than marine catfish, the bottom longline fishery targeting marine catfish has greater profitability than the bottom traps fishery targeting lobster (Tirtadanu et al., 2024a). Increased fishing efforts without proper management may result in population decline and significant economic losses. Moreover, the giant catfish fishery in Gunungkidul waters has contributed economically to a marketing chain that includes intermediaries, traders, restaurants, and processing industries; thus, the risk of population decline will adversely affect various fisheries businesses (Sarwanto et al., 2014). Therefore, the biological aspects and stock status of N. thalassina must be investigated as a basis for developing sustainable management measures for catfish fisheries.
Studies on the giant catfish fishery in Indonesia are still limited. Prior studies have reported that N. thalassina in the northern waters of Java shows carnivorous traits, prey on other fish, and it has a negative allometry growth pattern with the coefficient of b as 2.79 (Marbun et al., 2017; Taunay et al., 2013). N. thalassina has been found in the waters of the Arafura Sea, where the highest density in brackish environments characterized by shallow depths (Tirtadanu et al., 2024b). The reproductive and growth aspects of N. thalassina remain unexplored in Indonesia. The overexploited condition of catfish fisheries has been examined in Banyuasin waters through a production and effort approach (Fauziyah et al., 2020). Assessing stock status should incorporate the biological and life history of the target species to gain insights into its vulnerability to fishing pressure.
The stock assessment method applying production and effort data in Gunungkidul waters is difficult due to the lack of capacity to continuously record various fishing efforts and the risk of unreported production data linked to the dispersed landing sites of fishers in Gunungkidul. A length-based stock assessment is one of the methods that can be applied to the catfish fishery in Gunungkidul waters. This study investigated various biological aspects of N. thalassina, including fecundity, stomach contents, selectivity, length at first maturity, length-weight relationship, and growth. The stock status was determined by using multiple methods, including length-converted catch curve (LCC), yield per recruit (YPR), and spawning potential ratio (SPR) analysis (Hordyk et al., 2016; Sparre & Venema, 1992; Thompson & Bell, 1934). The uncertainty on the stock assessment was determined according to various natural mortality approaches. This study provides the first comprehensive assessment of biological characteristics and stock status of N. thalassina in the Gunungkidul waters, Indonesia. This study investigated the biological characteristics and stock status of the N. thalassina fishery as a basis for developing sustainable management strategies for the catfish fishery.
Materials and Methods
Data were collected from March to October 2023 in Baron landing site, with the coordinate of –8.12877°S and 110.54874°E. The catfish samples were collected from the catch of longline fishery operating in the southern waters of Gunungkidul, Baron Beach, and nearby coastal areas (Fig. 1). Salinity in the sampling area ranged from 21‰ to 26‰. The longline fishery was characterized by the one day fishing trip, using vessels with the sizes of 3 GT. The mean catch for the catfish by bottom longline was about 25 kg–1 vessel trip–1. The vessel was motorized with 15 hp (horsepower), and the fishing ground was less than 12 miles from the landing area. Each vessel deployed approximately 800 hooks per set. Bottom longlines were typically set in the afternoon and hauled at night, with a soak time of approximately 4 hours. Fishers typically made 18 to 25 fishing trips per month. A total of 516 catfish were sampled. Biological data collected from each specimen included length (cm), weight (g), sex, gonad maturity stage (using a defined scale), stomach contents (analyzed for prey composition), and fecundity (for mature females).
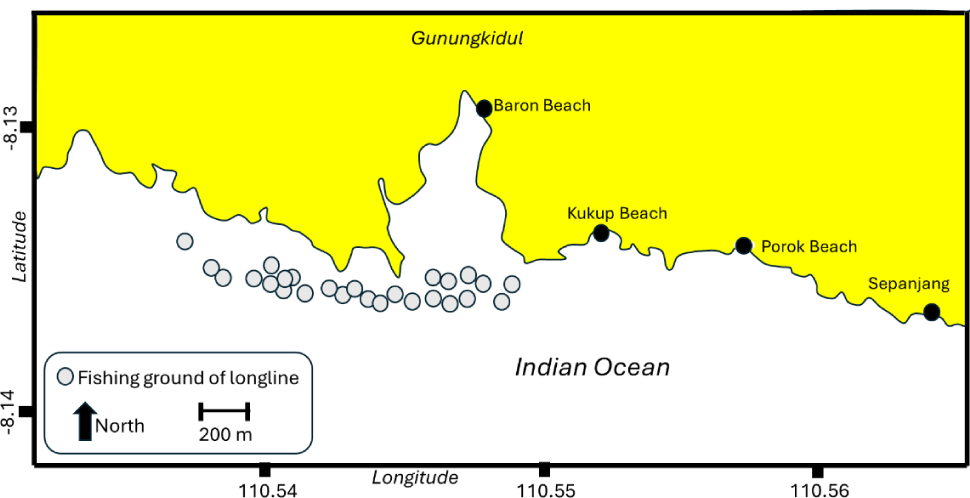
Biological characteristics of N. thalassina included size distribution, length at first capture (Lc50), length at first maturity (Lm50), stomach contents, fecundity, growth, and mortality parameters. Stock status was explored using the LCC approach, YPR analysis, and SPR.
Length-frequency data were grouped into 2 cm intervals and presented as a histogram to visualize the size structure of the population. Length at first capture and length at first maturity were estimated using a logistic regression (Sparre & Venema, 1992). The length-weight relationship was estimated using the power function (Ricker, 1975):
where a is a constant, b is the growth coefficient, L is fork length (cm), and W is the observed weight (g). The growth coefficient of b was calculated using a 95% confidence interval, representing the range within which the true value is likely to fall with 95% probability.
The relative condition factor (Kn) was estimated based on le Cren (1951):
where Kn is is the relative condition factor, W is the observed weight and W’ is the calculated weight.
Stomach contents were analyzed using the Index of Preponderance (IP) (Natarajan & Jhingran, 1961) to determine the relative importance of different prey items in the diet.
where IPi is an index of preponderance, Wi represents the contribution of each prey item to the total weight of stomach contents, while Oi reflects the frequency with which each prey item was observed in the stomachs examined.
Fecundity was determined by direct counts of oocytes from the ovaries of mature female catfish to estimate the reproductive potential of the species. A non-parametric LOESS smoother was used to describe the relationship between fecundity, length, and weight, allowing for visualization of the trend without assuming a specific functional form.
The growth parameters, including asymptotic length (L∞) and growth rate (K), were estimated using the electronic length frequency analysis with simulated annealing (ELEFAN SA) method within the TropFishR package (version 1.6.4) of the R program (R version 4.4.0) (Mildenberger et al., 2017; R Core Team, 2008; Xiang et al., 2013). A moving average value of 7 was performed to smooth the length frequency data and identify the fish growth over time. The theoretical age at which the fish attains a length of zero (t0) was calculated using equation (Pauly, 1983):
The asymptotic weight (W∞) was estimated from the cubic equation in the length weight relationship (Ricker, 1975):
where W∞ is the asymptotic weight (g), L∞ is asymptotic length (cm), a is the constant and b is the coefficient growth.
The maximum age (tmax) was measured by the equation (Alagaraja, 1984):
where tmax is the maximum age (years), K is the growth rate (year–1), and L∞ is asymptotic length (cm).
Natural mortality (M) was estimated using multiple empirical models to account for uncertainty in the stock assessment, as M is a critical parameter that is difficult to estimate directly. The five empirical natural mortality methods were selected based on the suitability of the available data and the life history characteristics of the species. The estimation of natural mortality was assessed using the following equation:
M1, M2, M3, M4 and M5 represent the natural mortality (year–1) estimates derived from the methodologies of Alagaraja (1984), Alverson & Carney (1975), Jensen (1997), Then et al. (2015), and Zhang & Megrey (2006), respectively. tmax represents the maximum age (year–1), K denotes the growth rate (year–1), β indicates the coefficient growth derived from the length-weight relationship, Ci is a constant valued at 0.44 that corresponds to demersal fish, t0 refers to the theoretical age at which N. thalassina has zero length (year), and L∞ is the asymptotic length (cm).
Total mortality (Z) was estimated using the LCC method as described by Sparre & Venema (1992). Fishing mortality (F) was estimated as the difference between Z and natural mortality (M): F = Z – M. This assumes that total mortality is solely due to natural mortality and fishing mortality. The exploitation rate was calculated using the ratio of fishing mortality to total mortality (Gulland, 1983).
Length-based YPR analyses were performed by the Thompson and Bell model through the TropFishR package in the R program (Mildenberger et al., 2017; R Core Team, 2008; Thompson & Bell, 1934). The fishing mortality corresponding to the maximum YPR (Fmax) was applied as the limit reference point (LRP), while the fishing mortality that produced a YPR of 10% of the initial biomass (F0.1) was designated as the target reference point in the YPR analysis (King, 1995).
The SPR was calculated using the ratio of spawning stock biomass per recruit in both exploited and unfished stocks (Goodyear, 1993; Hordyk et al., 2016). The SPR is determined by several input parameters: asymptotic length (L∞), growth rate (K), lengths at first captured (Lc50), length at 95% capture probability (Lc95%), length at first maturity (Lm50), length at 95% maturity (Lm95), natural mortality (M), and current fishing mortality (F). The SPR was illustrated as a function of fishing mortality (F) by using variation of natural mortality to account for uncertainty. Each value from the five natural mortality methods was used as an input parameter to understand the sensitivity of the SPR. The biological reference points utilized in the SPR analysis included fishing mortality corresponding to 20% SPR as the LRP and 40% SPR as the target reference point. A SPR less than 20% indicates a very low percentage of the spawners in nature, potentially disrupting recruitment (Gabriel & Mace, 1999). Moreover, N. thalassina inhabit a limited environment and have a low fecundity; the low proportion of spawners will lead to population loss (Parab, 1998; Tirtadanu et al., 2024b). As a conservative metric for population sustainability, the expected SPR is 40% (Clark, 2002). This justifies using 20% SPR as the LRP and 40% SPR as the target reference point.
Results
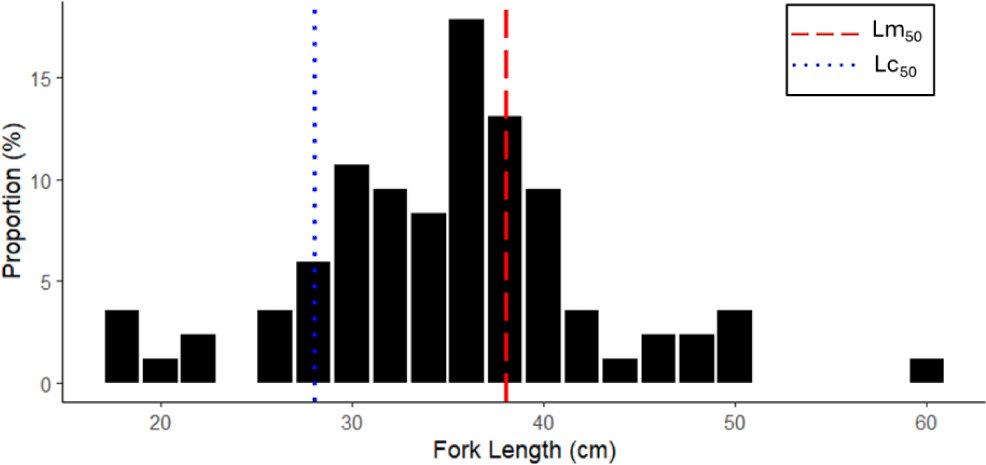
A total of 516 catfish’s length data were collected during March to December 2023. The length of N. thalassina captured by bottom longline in Gunungkidul waters ranged from 18 to 60 cm (Fig. 2). The average length was 35.44 ± 7.49 cm (± SD). The modal size of the catch was 38 cm. The length at first capture (Lc50 = 29 cm) was smaller than the length at first maturity of N. thalassina (Lm50 = 37 cm), indicating that the majority of the fish were captured before reaching gonadal maturity. Approximately 63% of the fish caught were below the length at first maturity, indicating a high proportion of immature individuals in the catch.
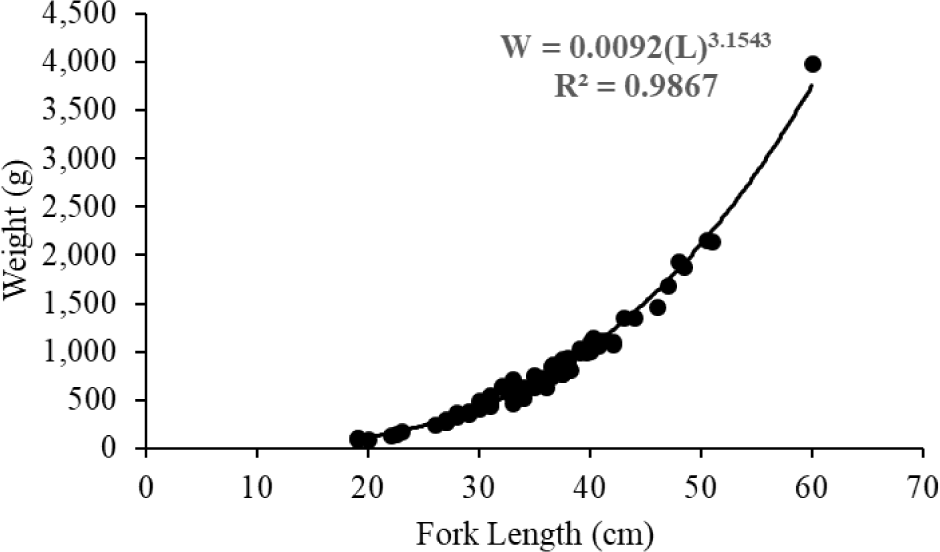
The weight of N. thalassina ranged from 96 to 3,980 g, with a mean weight of 808 ± 555 g (± SD). The length of N. thalassina ranged from 19 to 60 cm, with a mean length of 35.44 ± 7.49 cm. The findings indicated that the length-weight relationship followed the equation W = 0.09 (L)3.1543 (Fig. 3). According to the 95% confidence interval of b, the growth coefficient ranged from 3.07 to 3.24, indicating that the growth pattern of N. thalassina has a positive allometric pattern. The relative condition factor (Kn) of N. thalassina was 1.00 ± 0.09 (Mean ± SD), indicating that the catfish’s body weight is optimal. According to the length-weight equation, the length at first maturity (Lm50 = 37 cm) corresponded to a weight of 818 g, whereas the length at first capture (Lc50 = 29 cm) corresponded to a weight of 379 g.
The stomach contents of N. thalassina in Gunungkidul waters consisted of crabs, stomatopods, shrimps, and fish (Table 1). Among the 21 stomach samples of N. thalassina, crabs had the highest occurrence percentage at 57%, followed by stomatopods at 33%. The highest and second highest percentages of food weight and index of preponderance in the stomach contents of N. thalassina were found in crabs and stomatopods. The index of preponderance for crabs was 60.11%, while for stomatopods, it was 29.96%. The index of preponderance of shrimps and fishes was below 10%.
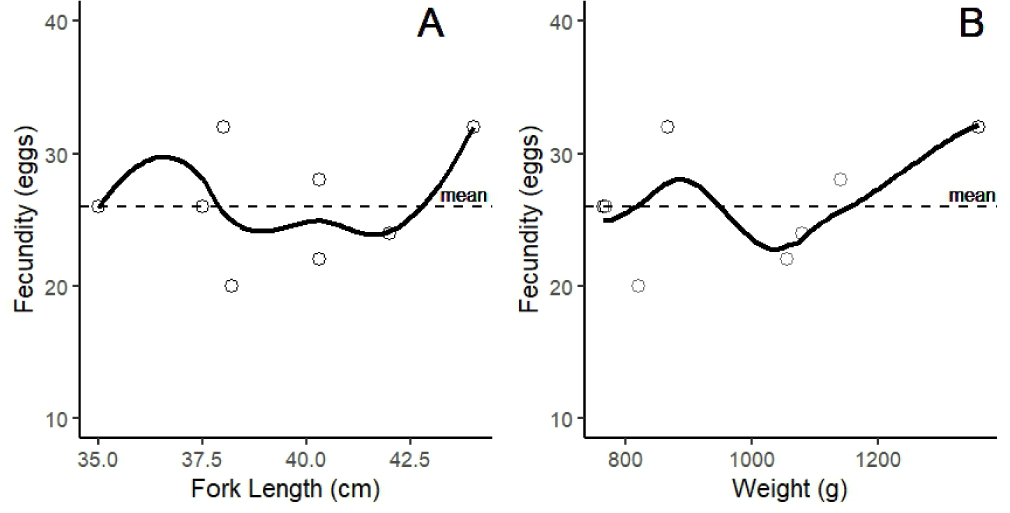
Fecundity observations of female N. thalassina indicated a low fecundity. The number of eggs ranged from 20 to 32, with an average of 26 ± 4 eggs (± SD) (Fig. 4). The findings indicated an absence of a linear correlation between fecundity and the length and weight of N. thalassina. The peak fecundity was observed at a length of 44 cm and a weight of 1.359 g.
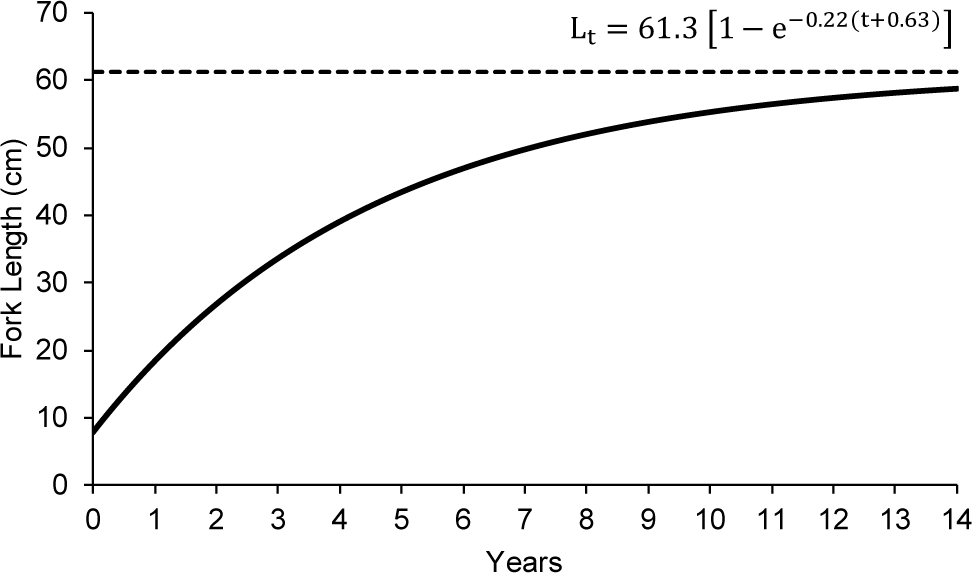
The output from TropFish R, based on the monthly length frequency data, indicated that the asymptotic length (L∞) of N. thalassina was 61.30 cm, with a growth rate (K) of 0.22 year–1 and a relative fitness score (Rn) of 0.57 (Fig. 5). The theoretical age of N. thalassina at length 0 (t0) was –0.63. Therefore, the Von Bertalanffy equation for N. thalassina in Gunungkidul waters was represented as:
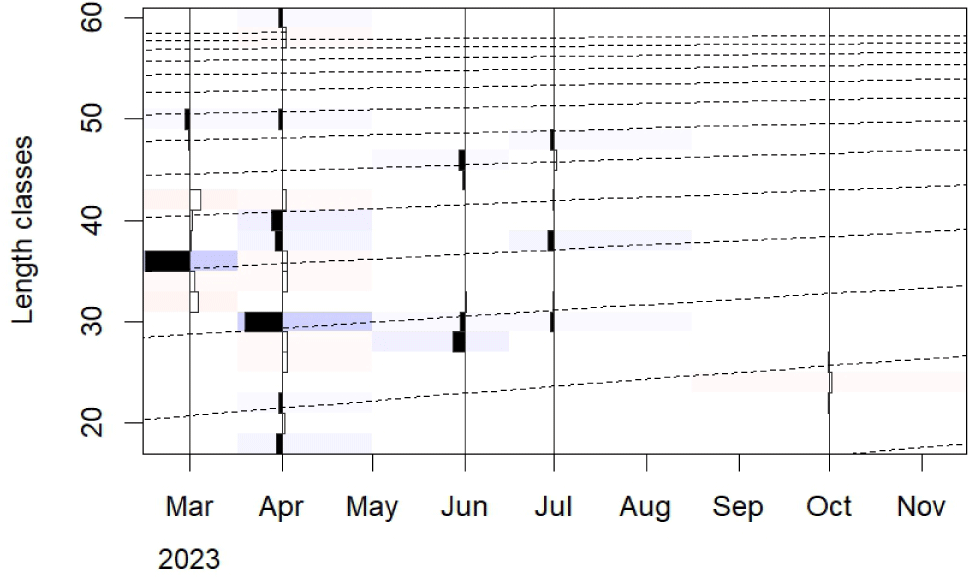
The asymptotic length (L∞) of N. thalassina was 61.30 cm, corresponding to an asymptotic weight (W∞) of 4,019 g. The maximum age (tmax) of N. thalassina was 13 years. The length at first maturity (Lm50) of 37 cm was attained at 3 years of age (Fig. 6). The length at first capture (Lc50) was approximately 2 years and 4 months of age.
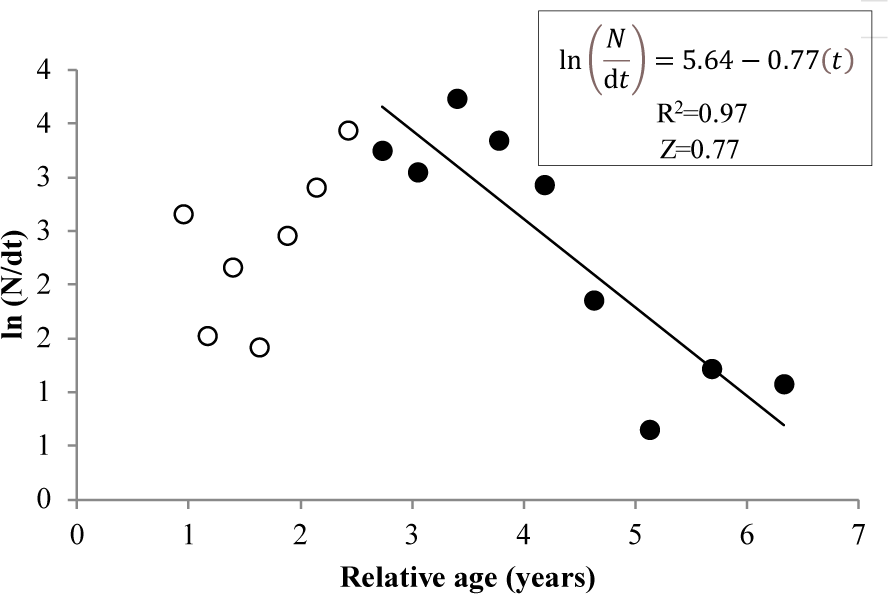
The total mortality rate of N. thalassina in Gunungkidul waters was 0.77 year–1 (Fig. 7). The average natural mortality of N. thalassina, varied from five natural mortality approaches, was 0.35 ± 0.09, which corresponds to a fishing mortality of 0.42 ± 0.09 (± SD) (Table 2). The exploitation rates of N. thalassina ranged from 0.39 and 0.70. The natural mortality estimation from Then et al. (2015) (MTh = 0.47 year–1) led to the lowest exploitation rate of 0.39, whereas the natural mortality from Zhang & Megrey (2006) (MZM = 0.23) produced the highest exploitation rate of 0.70. The average current exploitation rate was 0.55 ± 0.11, indicating that the current status of giant catfish fishery is in the fully exploited condition.
| Methods of M | Z (year–1) | M (year–1) | F (year–1) | E (year–1) |
|---|---|---|---|---|
| Alverson & Carney (1975) | 0.77 | 0.34 | 0.43 | 0.56 |
| Alagaraja (1984) | 0.77 | 0.35 | 0.42 | 0.55 |
| Jensen (1997) | 0.77 | 0.33 | 0.44 | 0.57 |
| Zhang & Megrey (2006) | 0.77 | 0.23 | 0.54 | 0.70 |
| Then et al. (2015) | 0.77 | 0.47 | 0.30 | 0.39 |
| Mean ± SD | 0.77 | 0.35 ± 0.09 | 0.42 ± 0.09 | 0.55 ± 0.11 |
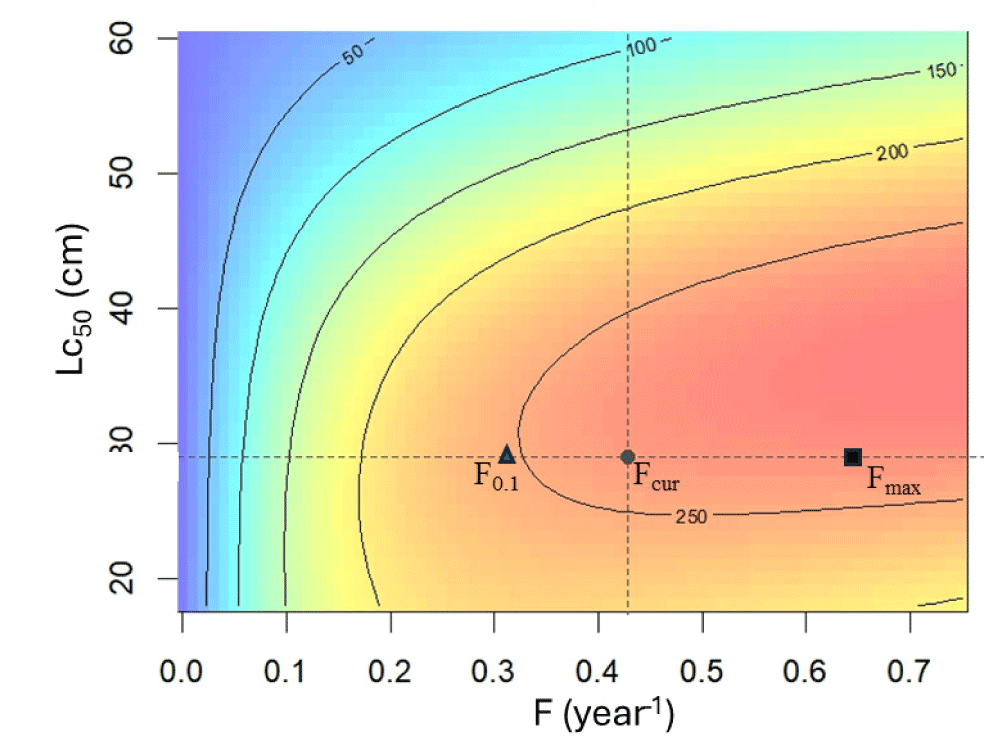
By applying the average fishing mortality rate of 0.42 year–1, the current YPR of N. thalassina (YPRcur) is 259.85 g recruit–1 (Fig. 8). The YPR (YPRmax) will attain a peak of 268.63 g recruit–1 if fishing mortality rises to a maximum fishing mortality (Fmax) of 0.64 year–1. However, the current fishing mortality (Fcur) has exceeded 26% of the reference point F0.1. Reference point F0.1 was 0.31 year–1, yielding 247 grams recruit–1. The current fishing mortality lies between the LRP Fmax and the target reference point F0.1, indicating a fully exploited condition.
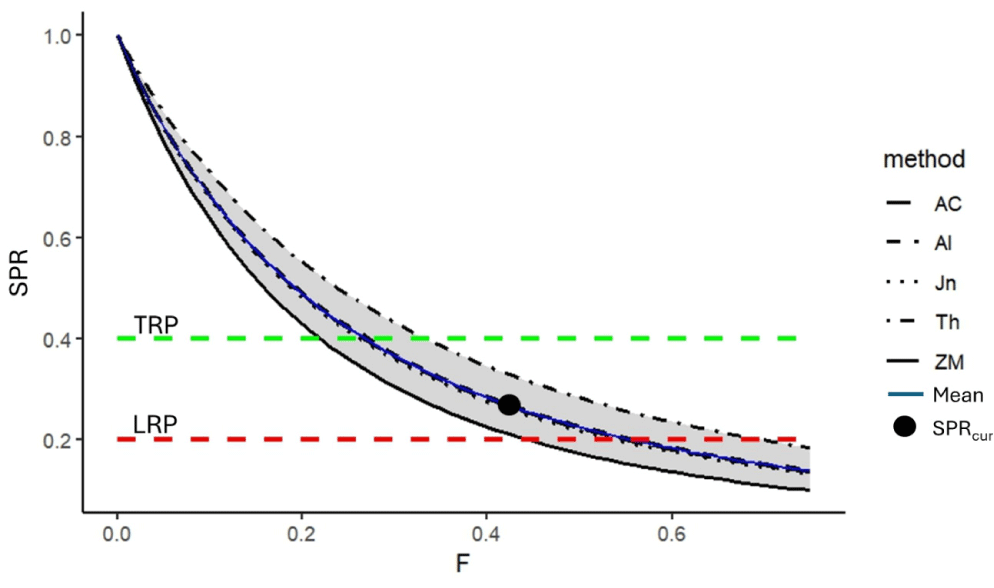
By using variations of the four natural mortality methods, the SPR of N. thalassina in Gunungkidul waters ranged from 0.22 to 0.34 (Table 3). The low natural mortality value from Zhang & Megrey (2006) (M = 0.23) implies a low SPR estimate of 0.22. In contrast, the largest estimate of SPR of 0.34 was obtained using the largest estimate of natural mortality from Then et al. (2015). However, the variation of the SPR, with an average of 0.28 ± 0.05, is within the range of the LRP of 20% SPR and the target reference point of 40% SPR, indicating that the spawning stock biomass is in the precautionary condition (Fig. 9). Fishing mortality resulting in a 40% SPR (F40%) is 33% less than current fishing mortality, so a 33% reduction in fishing effort would shift the status to the sustainable condition.
AC, Al, Jn, ZM and Th as the natural mortality (M) by Alverson & Carney (1975), Alagaraja (1984), Jensen (1997), Then et al. (2015), Zhang & Megrey (2006).
Discussion
Most N. thalassina captured in Gunungkidul waters were small or below the length at first maturity (< Lm50). The study indicated that 63% of the fish population consisted of small fish (< Lm50). N. thalassina captured by bottom longlines in Gunungkidul waters ranged from 18 to 60 cm, close to those from the Oman Sea, which ranged from 17 to 64 cm. However, N. thalassina in the current study were smaller than those of N. thalassina caught by gillnet in Vietnamese waters, which ranged from 51 to 106 cm (Nguyen, 2024; Vahabnezhad et al., 2021). This condition may be affected by the dominant capture of small-sized fish that cannot reach their maximum size. The length at first capture of N. thalassina by the bottom longline (Lc50 = 29 cm) was smaller than the length at first maturity (Lm50 = 37 cm), indicating that the fishery is catching fish before they have had a chance to reproduce, which can have negative consequences for the population. This condition causes a low percentage of spawners in nature, which will lead to population loss. Consequently, it is essential to prevent capturing small fish to facilitate their growth and reproduction. Previous studies indicated that the minimum legal size corresponds to the length at first maturity (Tirtadanu et al., 2023; Widiyastuti & Tirtadanu, 2024). Raising the length at first capture from 29 cm to 37 cm may serve as a management option to conserve N. thalassina in Gunungkidul waters. The government can support this initiative by replacing small hook sizes with larger sizes.
The growth pattern of N. thalassina in Gunungkidul waters showed positive allometry, characterized by a growth coefficient b of 3.15, which indicated a relatively fat body condition. Moreover, the catfish’s body weight condition is good, with the relative condition factor (Kn) of 1.00. A similar condition of growth pattern was observed in the waters of Kuwait, Cilacap-Indonesia and Bangladesh whereas a negative allometric pattern was found for N. thalassina in the Vietnam, Pekalongan-Indonesia and Pakistan waters (Table 4). The results indicated that the habitat of Gunungkidul waters remains suitable for the growth of N. thalassina, with sufficient food resources.
| Species | Length-weight relationship | Areas | Source | |
|---|---|---|---|---|
| a | b | |||
| Netuma bilineata | 0.0051 | 3.16 | Kuwaiti Waters | Al-Husaini et al. (2021) |
| Netuma thalassina | 0.032 | 2.76 | Vietnam | Nguyen (2024) |
| Netuma thalassina | 0.0158 | 3.05 | Cilacap Waters | Anggawangsa & Faizah (2020) |
| Netuma thalassina | 0.00003 | 2.79 | Pekalongan Waters | Marbun et al. (2017) |
| Netuma thalassina | 0.011 | 2.89 | Arabian Coast, Pakistan | Farooq et al. (2017) |
| Netuma thalassina | 0.004 | 3.29 | Bay of Bengal, Bangladesh | Sultana et al. (2019) |
| Netuma thalassina | 0.0092 | 3.15 | Gunungkidul waters, Indonesia | Present study |
This study revealed that N. thalassina is a carnivore, consuming various food types such as crabs, stomatopods, shrimps, and fish. The primary diet of N. thalassina in Gunungkidul waters consists of crabs and stomatopods (mantis shrimps), with a preponderance index proportion of 60.11% and 29.96%, respectively. The primary food source is significantly affected by food availability within its habitat. The predominant components of the stomach contents of N. thalassina in Omani waters were bivalves and gastropods, whereas in Semarang waters, the primary dietary component was fish (Taunay et al., 2013; Vahabnezhad et al., 2021). The optimal fatness condition of N. thalassina in Gunungkidul waters indicates abundant preys, such as a population of crabs and stomatopods in the benthic zone of this region.
N. thalassina in Gunungkidul waters has low fecundity, ranging from 20 to 32 eggs. Parab (1998) reported low fecundity of N. thalassina in Indian waters, with a fecundity range of 34 to 88 eggs. The fecundity of N. thalassina is significantly lower than that of other demersal fisheries commodities, such as Nemipterus spp. and Saurida spp., which can produce from hundreds to thousands of eggs (İşmen, 2003; Rahman & Samat, 2021). Moreover, N. thalassina in Gunungkidul waters has a slow growth rate, with a K value of 0.22 year–1 and a maximum lifespan of 13 years. Al-Husaini et al. (2021) reported a slightly faster growth rate of 0.30 year–1 for N. thalassina in Kuwaiti waters. The lowest growth rate value of N. thalassina was found in India, with K value as 0.20 (Table 5). The low fecundity and relatively small growth rate indicated that N. thalassina is susceptible to population decline due to overfishing. N. thalassina populations must be allowed to grow and reproduce to enhance recruitment levels. N. thalassina reached gonad maturity at 3 years, with a maximum lifespan of 13 years, indicating a relatively long reproductive period if smaller individuals were not harvested. Management practices prioritizing a landing size greater than 37 cm are strongly recommended to ensure the sustainability of N. thalassina in the waters of Gunungkidul.
| Species | L∞ (cm) | K (year–1) | M (year–1) | Areas | Sources |
|---|---|---|---|---|---|
| Netuma bilineata | 46.5a | 0.35 | - | Kuwaiti waters | Al-Husaini et al. (2021) |
| Netuma thalassina | 91.6a | 0.30 | - | Kuwaiti waters | Al-Husaini et al. (2021) |
| Netuma thalassina | 97.6a | 0.33 | 0.62 | Bengal Coast, Bangladesh | Sultana et al. (2019) |
| Netuma thalassina | 84.8a | 0.20 | - | Mandapam Waters, India | Menon (1986) |
| Netuma thalassina | 68.0a | 0.45 | 0.84 | Brunei Darussalam | Silvestre & Garces (2004) |
| Netuma thalassina | 61.3b | 0.22 | 0.35 | Gunungkidul waters, Indonesia | Present study |
The stock assessment of the N. thalassina fishery should consider a precautionary approach due to its status as a commercial commodity characterized by low fecundity and relatively slow growth rate, which increases its vulnerability to stock decline. This study presented fisheries stock assessment using multiple methods: LCC, YPR, and SPR analysis. The LCC method indicated variations in several natural mortality calculation approaches, resulting in exploitation rates (E) from 0.39 to 0.70, with a mean of 0.55 ± 0.11. The average exploitation rate of N. thalassina in Gunungkidul waters is classified as fully exploited (yellow zone), aligning with the optimal exploitation rate of 0.5 proposed by Gulland (1983). This status resembles the results of the YPR and SPR analysis, finding that the current fishing mortality lies between the LRP Fmax and the target reference point F0.1. Based on the length-based SPR, the current fishing mortality lies between the LRP of 20% SPR and the target reference point of 40% SPR.
Applying several approaches for assessing stock status is important for comprehending uncertainty and informing policy decisions by fisheries managers. Using the natural mortality approach from Then et al. (2015) resulted in a more optimistic assessment of the N. thalassina fishery in Gunungkidul waters, impacting a lower exploitation rate and a higher SPR. In contrast, the natural mortality method from Zhang & Megrey (2006) provided a more conservative estimate, leading to a higher exploitation rate and a low SPR. By understanding the biological characteristics of N. thalassina, decision-making should focus on achieving the target reference point. A SPR of 40% for N. thalassina is recommended due to its dominant small-size capture (63% below length at first maturity), low fecundity, and slow growth rate. The fishing mortality rate of N. thalassina currently surpasses 33% of the target reference point F40%. Therefore, a 33% reduction in fishing efforts is strongly advised to ensure the sustainability of the N. thalassina fishery in Gunungkidul waters. Fishing effort reduction can be achieved through the socialization of optimal hook-size fishing gear and government-supported hook-size modifications. Enhancing the SPR can improve the reproduction and recruitment of N. thalassina in natural environments, leading to the sustainability of the N. thalassina fishery.
Conclusion
The findings indicated that the majority of giant catfish (N. thalassina_ captured by longline in Gunungkidul were small-size fish, as evidenced by the length at first capture (Lc = 29 cm), which is below the length at first maturity (Lm50 = 37 cm). N. thalassina has a relatively fat body shape and is carnivorous, consuming various food items, including crabs, stomatopods, shrimps, and fish. Crabs constituted the primary diet, indicating from a high preponderance index of 60%. Fecundity was low, with a mean of 26 ± 4 eggs (± SD). The asymptotic length (L∞) and growth rate (K) were 61.3 cm and 0.22 years–1, respectively. The status of the N. thalassina fishery is classified as fully exploited. The fishing mortality of N. thalassina currently surpasses 33% of target reference point F40%. Therefore, a 33% reduction in effort is strongly recommended to ensure the sustainability of N. thalassina fishery in Gunungkidul waters. Effort reduction can be achieved through the socialization of optimal hook-size fishing gear and government-supported changes in hook-size. Raising the SPR is recommended to improve the reproduction and recruitment of N. thalassina in natural environments, thereby influencing the future viability of the N. thalassina fishery.








